|
There are several types of fuel cells, each of which is
generally defined by there operating temperature and the type of
electrolyte they use.
The choice of which type to
use is often based on the targeted application of the fuel cell.
For example, some types of fuel cells work well for use in
stationary power generation
plants while others may be useful for
powering cars or small portable
applications.
The best-known types of fuel cells are alkaline
(AFC), molten carbonate (MCFC), phosphoric acid (PAFC), proton
exchange membrane (PEMFC) and solid oxide (SOFC). Each work on
the same principle, striping electrons from hydrogen atoms to
create electricity.
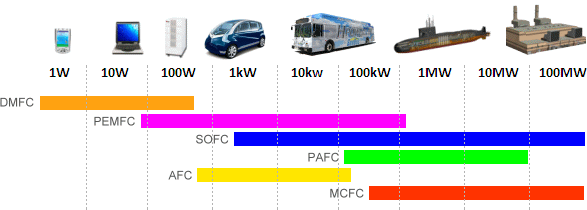
An example of the differences between
fuel cell types and there ideal target output
Proton Exchange Membrane (PEMFC)
PEM fuel cells use a platinum coated solid polymer that require
only hydrogen and oxygen to produce electricity.
The advantages of a PEM fuel cell is that they
have a high power density and are low in weight. They are also
able to operate in low temperatures, typically around 80ºC which
allows them to start start quickly (less warm uptime). The major
disadvantage of PEM fuel cells is that they require an expensive
catalyst, in this case platinum, which adds to the overall unit
cost. Hydrogen delivery is also another significant barrier is
it requires careful handling and storage.
This makes PEM fuel cells more applicable for industrial uses
where delivery and storage can be in a controlled environment.
Direct Methanol Fuel Cells (DMFC)
Direct Methanol Fuel Cells are PEM based fuel cell that use
methanol instead of pure hydrogen. As such, they share many of
the same advantages of a PEM fuel cell, with the added benefit
that methanol is a much easier and safer fuel for transportation
and storage. The disadvantage of methanol is that the
reaction is often not as efficient as pure hydrogen because
methanol can often move across the membrane without reacting
with the catalyst. To address these concerns, some fuel cells
reform the methanol (RMFC) before it reacts with the catalyst.
While this approach improves the efficiency of methanol, it
still requires an expensive reforming process that needs to
operate at much higher temperatures (250ºC)
to first burn-off the methanol in order to release hydrogen.
Alkaline Fuel Cells (AFC)
Alkaline
Fuel Cell is one of the oldest types of fuel cell. The electrolyte
they use is made
of liquid potassium hydroxide. One of the major advantages for
an alkaline fuel cell is that non-precious metals can be used as
a catalyst. However, the catalysts can be easily poisoned by CO2
(carbon dioxide). As such, the hydrogen and oxygen used in an
AFC needs to be purified, which is a more costly process. The
operating temperature of AFC's is between 100ºC
~ 250ºC.
Solid Oxide Fuel Cells (SOFC)
Unlike other fuel cells, Solid Oxide Fuel Cell is
composed of entirely solid materials. The electrolyte is a hard
ceramic material made up mainly of zirconium oxide with small
amounts of ytrria. The systems operate at about 600ºC~1000ºC. The high temperature means these are
slow starting systems and most are designed for larger,
stationary applications in the 25 kW to 100 kW range
Phosphoric Acid Fuel Cells (PAFC)
Phosphoric acid is the electrolyte used in an PAFC. As an acid,
it requires a very high temperature(150ºC ~ 200ºC) to
start the reaction. It also uses platinum as a catalyst, similar
to PEM fuel cells, which is costly. However, PAFC's are more
tolerant to impurities in fuels than PEM fuel cells, and they
are very efficient at generating electricity and heat - which
makes them ideal for power plants. However, they are typically
large and heavy which makes them less than ideal for smaller or
portable applications.
|

